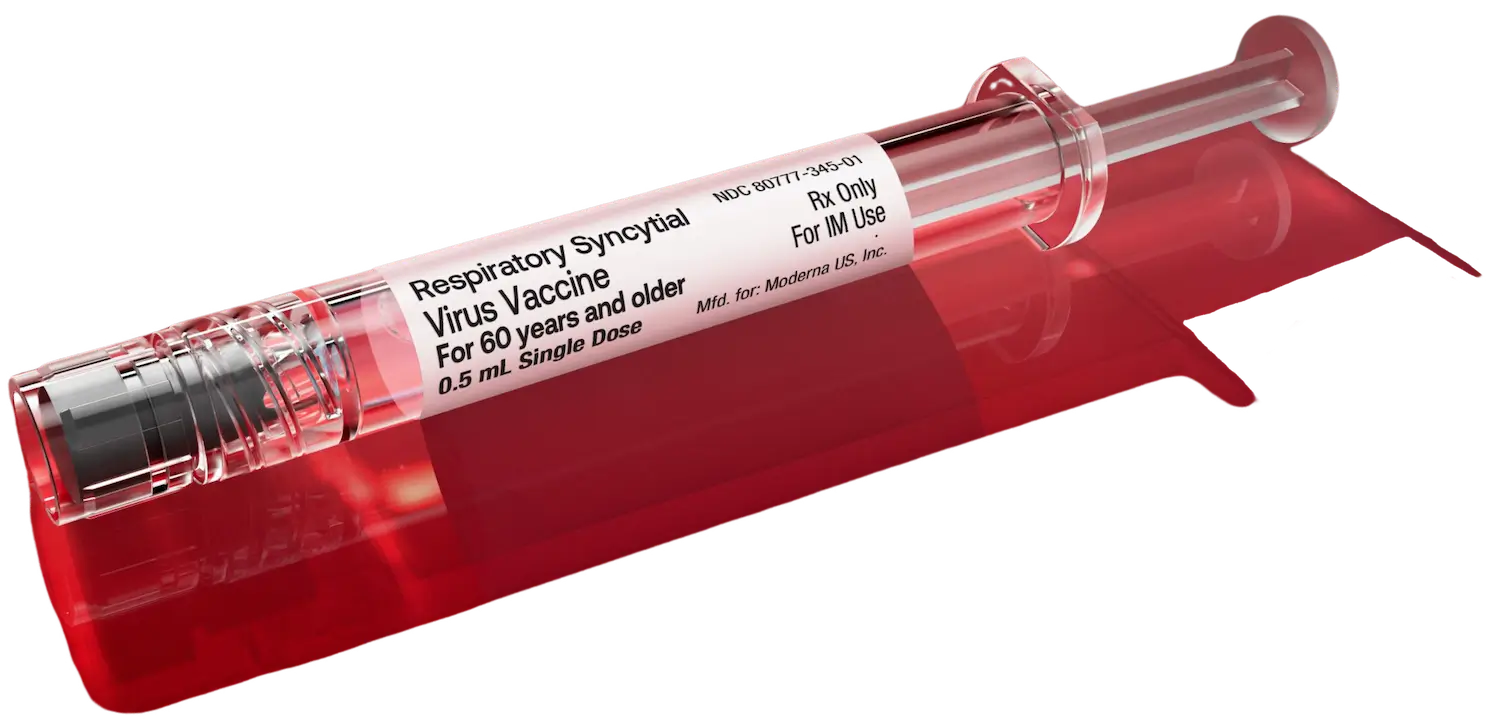
The only adult RSV protection available in a pre-filled syringe1
The only adult RSV protection available in a pre-filled syringe1
Ready to protect against RSV. No reconstitution required.1 mRESVIA is a single-dose 0.5 mL pre-filled syringe, ready for you to use for protection against RSV.1
Pre-filled syringes offer a convenient way to administer protection.1-3
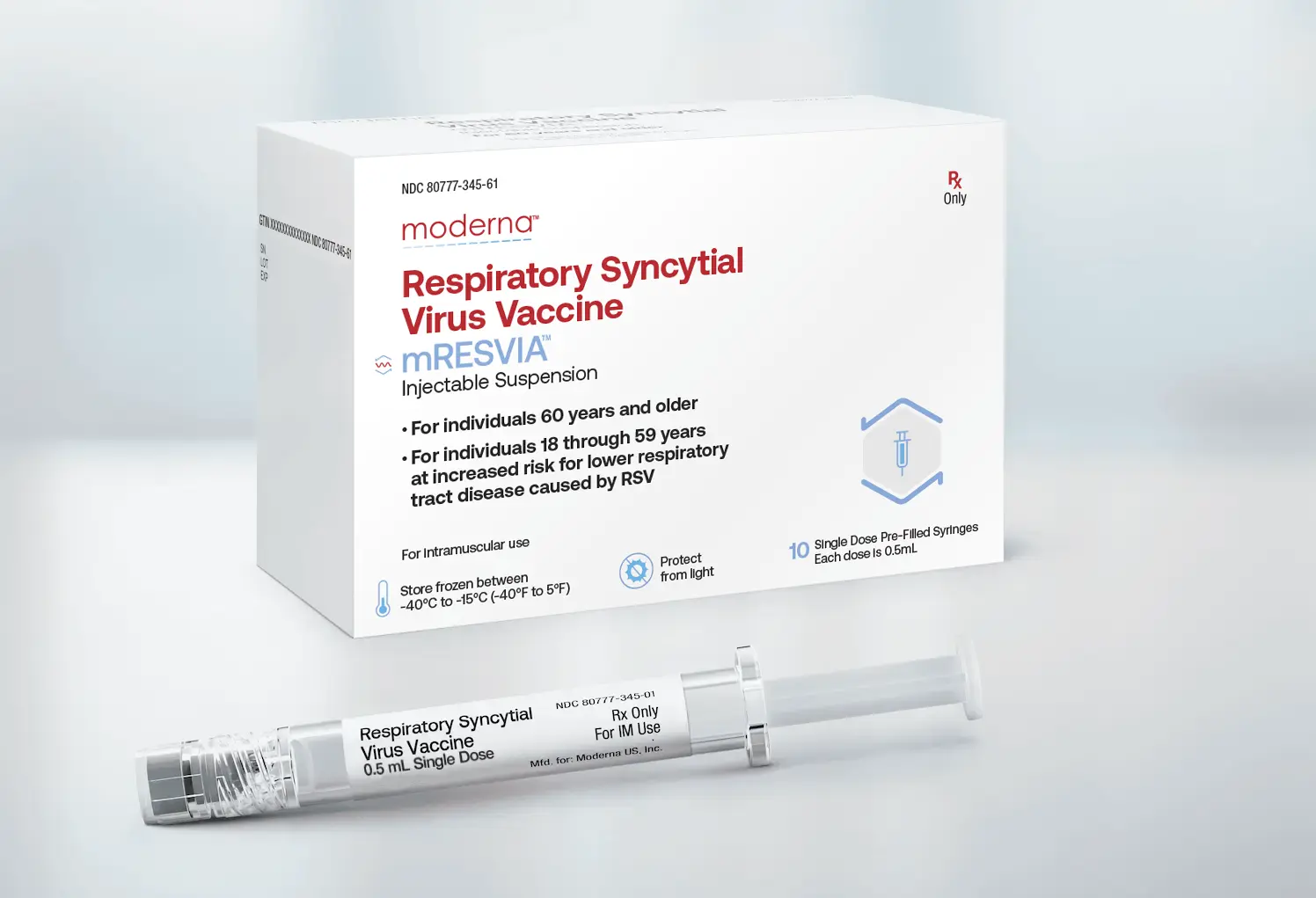
mRESVIA Carton of 10 Dimensions: 110 x 102 x 35mm
mRESVIA Carton of 2 Dimensions: 110 x 54 x 30mm
Storage and handling options1
Your mRESVIA pre-filled syringes can be stored in a freezer, refrigerator, or at room temperature, with varying storage times.1*

Store in the freezer
-40°F to 5°F (-40°C to -15°C)1

Store up to 90 days† in the refrigerator
36°F to 46°F (2°C to 8°C)1

Store up to 24 hours‡ at room temperature
46°F to 77°F (8°C to 25°C)1
Storage and administration considerations
- Do not shake mRESVIA1
- Avoid exposure to direct sunlight and/or ultraviolet light, and minimize exposure to room light1
- Do not refreeze after thawing or refrigerate after storing at room temperature1
Check out the mRESVIA Product Information Quick Guide
Check out the mRESVIA Product Information Quick Guide
*Discard any syringes left at room temperature for over 24 hours.1
†Following frozen storage.1 ‡Following refrigerated storage.1
LRTD = lower respiratory tract disease; RSV = respiratory syncytial virus.
INDICATION
mRESVIA® (Respiratory Syncytial Virus Vaccine) is a vaccine indicated for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older and individuals 18 through 59 years of age who are at increased risk for LRTD caused by RSV.
IMPORTANT SAFETY INFORMATION
Contraindications
Do not administer mRESVIA to individuals with a history of severe allergic reaction (e.g., anaphylaxis) to any component of mRESVIA.
Warnings and Precautions
- Management of Acute Allergic Reactions: Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of mRESVIA.
- Syncope: Syncope (fainting) may occur in association with administration of injectable vaccines, including mRESVIA. Procedures should be in place to avoid injury from fainting.
- Altered Immunocompetence: Immunocompromised individuals, including those receiving immunosuppressive therapy, may have a diminished immune response to mRESVIA.
Adverse Reactions
In a clinical trial conducted in participants 60 years of age and older, the most commonly reported (≥10%) adverse reactions were injection-site pain (55.9%), fatigue (30.8%), headache (26.7%), myalgia (25.6%), arthralgia (21.7%), axillary (underarm) swelling or tenderness (15.2%) and chills (11.6%).
In a clinical trial conducted in participants 18 through 59 years of age at increased risk for LRTD caused by RSV, the most commonly reported (≥10%) adverse reactions were injection site pain (73.9%), fatigue (36.9%), headache (33.3%), myalgia (28.9%), arthralgia (22.7%), chills (19.9%), axillary (underarm) swelling or tenderness (17.1%), and nausea/vomiting (10.8%).
To report suspected adverse reactions, contact ModernaTX, Inc. at 1-866-663-3762 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please click for mRESVIA Full Prescribing Information.References
- mRESVIA Prescribing Information. ModernaTX, Inc.
- Icardi G, Orsi A, Vitali Rosati G, Tognetto A, Checcucci Lisi G, Parisi S. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61(3):E424-E444. doi:10.15167/2421-4248/jpmh2020.61.3.1535
- Yarnoff B, Bodhaine S, Cohen E, Buck PO. Time and cost of administering COVID-19 mRNA vaccines in the United States. Hum Vaccin Immunother. 2021;17(11):3871-3875. doi:10.1080/21645515.2021.1974289
For Colorado and Connecticut price disclosure, please visit https://modernadirect.com/wac-disclosure
Stay up to date
Receive the latest information about mRESVIA and all the products in the Moderna pipeline.
By submitting your information, you agree to Moderna's Terms and Conditions. You also acknowledge that your information will be processed in accordance with Moderna's Privacy Statement. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
© 2026 Moderna US-RSV-2400107 01/2026