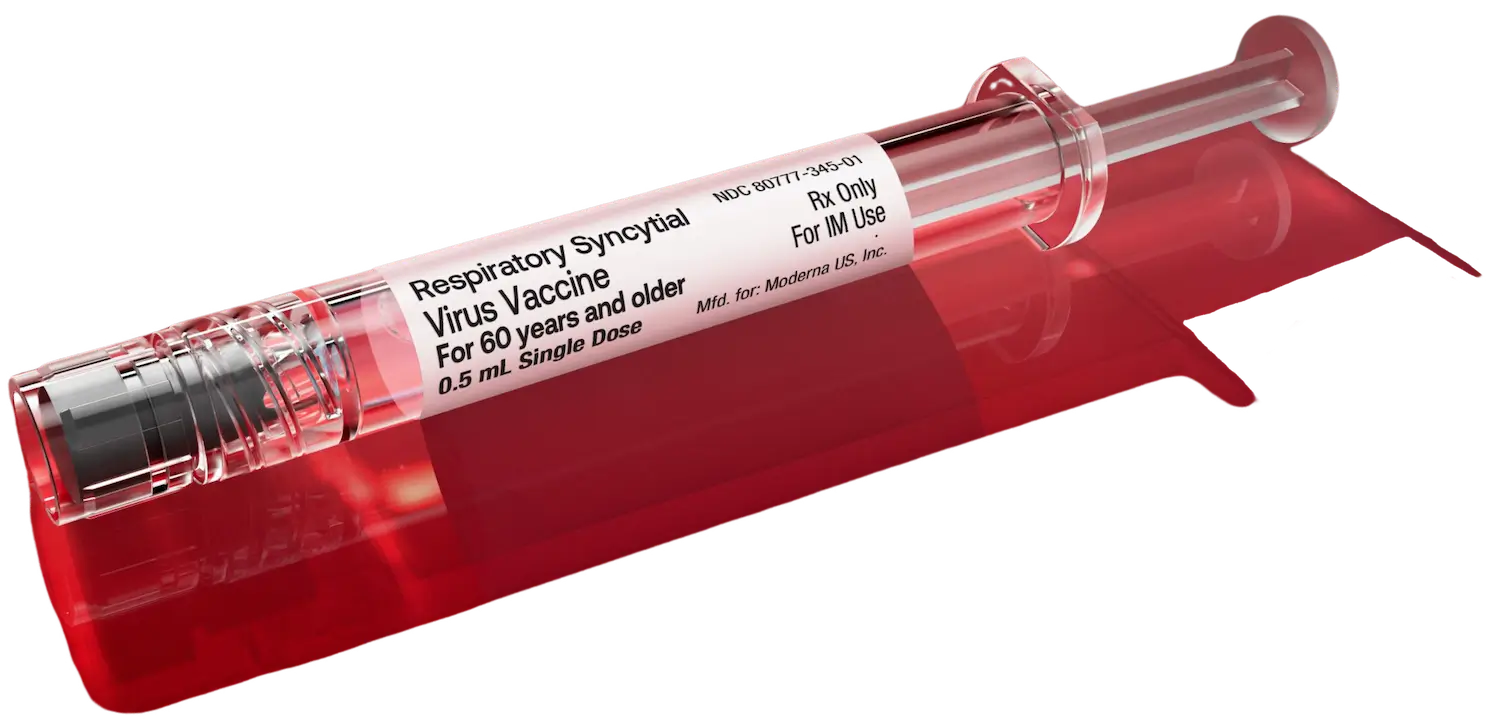
Exceptional convenience
Administration with a pre-filled syringe has its benefits.2

Proven efficacy1
See the results of the clinical trial.1
Explore the endpoints
A demonstrated safety profile1
Talking to your adult patients about RSV
Patients aged 60 or older and patients aged 18 to 59 who are at increased risk for LRTD caused by RSV may not realize the risks associated with RSV. You can help them understand what's at stake.
- There is no treatment for an RSV infection beyond supportive care3,4
- Studies have shown that adults with underlying chronic medical conditions, regardless of age, should be considered at risk of severe RSV-associated outcomes.5,6

CDC Recommendations for RSV Vaccines7-9
To protect against RSV, the CDC recommends vaccinating all adult patients who are:
- 50-74 years of age who are at increased risk of severe RSV disease7-9
- Aged 75 years or older8
Talk to your eligible adult patients to determine if mRESVIA is right for them.
LRTD = lower respiratory tract disease; RSV = respiratory syncytial virus.
INDICATION
mRESVIA® (Respiratory Syncytial Virus Vaccine) is a vaccine indicated for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals 60 years of age and older and individuals 18 through 59 years of age who are at increased risk for LRTD caused by RSV.
IMPORTANT SAFETY INFORMATION
Contraindications
Do not administer mRESVIA to individuals with a history of severe allergic reaction (e.g., anaphylaxis) to any component of mRESVIA.
Warnings and Precautions
- Management of Acute Allergic Reactions: Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of mRESVIA.
- Syncope: Syncope (fainting) may occur in association with administration of injectable vaccines, including mRESVIA. Procedures should be in place to avoid injury from fainting.
- Altered Immunocompetence: Immunocompromised individuals, including those receiving immunosuppressive therapy, may have a diminished immune response to mRESVIA.
Adverse Reactions
In a clinical trial conducted in participants 60 years of age and older, the most commonly reported (≥10%) adverse reactions were injection-site pain (55.9%), fatigue (30.8%), headache (26.7%), myalgia (25.6%), arthralgia (21.7%), axillary (underarm) swelling or tenderness (15.2%) and chills (11.6%).
In a clinical trial conducted in participants 18 through 59 years of age at increased risk for LRTD caused by RSV, the most commonly reported (≥10%) adverse reactions were injection site pain (73.9%), fatigue (36.9%), headache (33.3%), myalgia (28.9%), arthralgia (22.7%), chills (19.9%), axillary (underarm) swelling or tenderness (17.1%), and nausea/vomiting (10.8%).
To report suspected adverse reactions, contact ModernaTX, Inc. at 1-866-663-3762 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please click for mRESVIA Full Prescribing Information.References
- mRESVIA Prescribing Information. ModernaTX, Inc.
- Icardi G, Orsi A, Vitali Rosati G, Tognetto A, Checcucci Lisi G, Parisi S. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61(3):E424-E444. doi:10.15167/2421-4248/jpmh2020.61.3.1535
- Malik S, Ahmad T, Muhammad K, Waheed Y. Respiratory syncytial virus infection: treatments and clinical management. Vaccines (Basel). 2023;11(2):491. doi:10.3390/vaccines11020491
- Gatt D, Martin I, AlFouzan R, Moraes TJ. Prevention and treatment strategies for respiratory syncytial virus (RSV). Pathogens. 2023;12(2):154. doi:10.3390/pathogens12020154
- Prasad N, Walker TA, Waite B, et al. Respiratory syncytial virus-associated hospitalizations among adults with chronic medical conditions. Clin Infect Dis. 2021;73(1):e158-e163. doi:10.1093/cid/ciaa730
- Njue A, Nuabor W, Lyall M, et al. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect Dis. 2023;10(11):ofad513. doi:10.1093/ofid/ofad513
- Britton A. Evidence to recommendations framework (EtR) RSV vaccination in adults aged 20–59 years. Published April 16, 2025.
- Centers for Disease Control and Prevention. CDC updates RSV vaccination recommendation for adults. Updated August 30, 2024. Accessed June 6, 2025. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/older-adults.html
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). ACIP recommendations. Updated May 15, 2025. Accessed July 3, 2025. https://www.cdc.gov/acip/vaccine-recommendations/index.html#cdc_toolkit_main_toolkit_cat_1-recent-meeting-recommendations
For Colorado and Connecticut price disclosure, please visit https://modernadirect.com/wac-disclosure
Stay up to date
Receive the latest information about mRESVIA and all the products in the Moderna pipeline.
By submitting your information, you agree to Moderna's Terms and Conditions. You also acknowledge that your information will be processed in accordance with Moderna's Privacy Statement. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
© 2026 Moderna US-RSV-2400107 01/2026